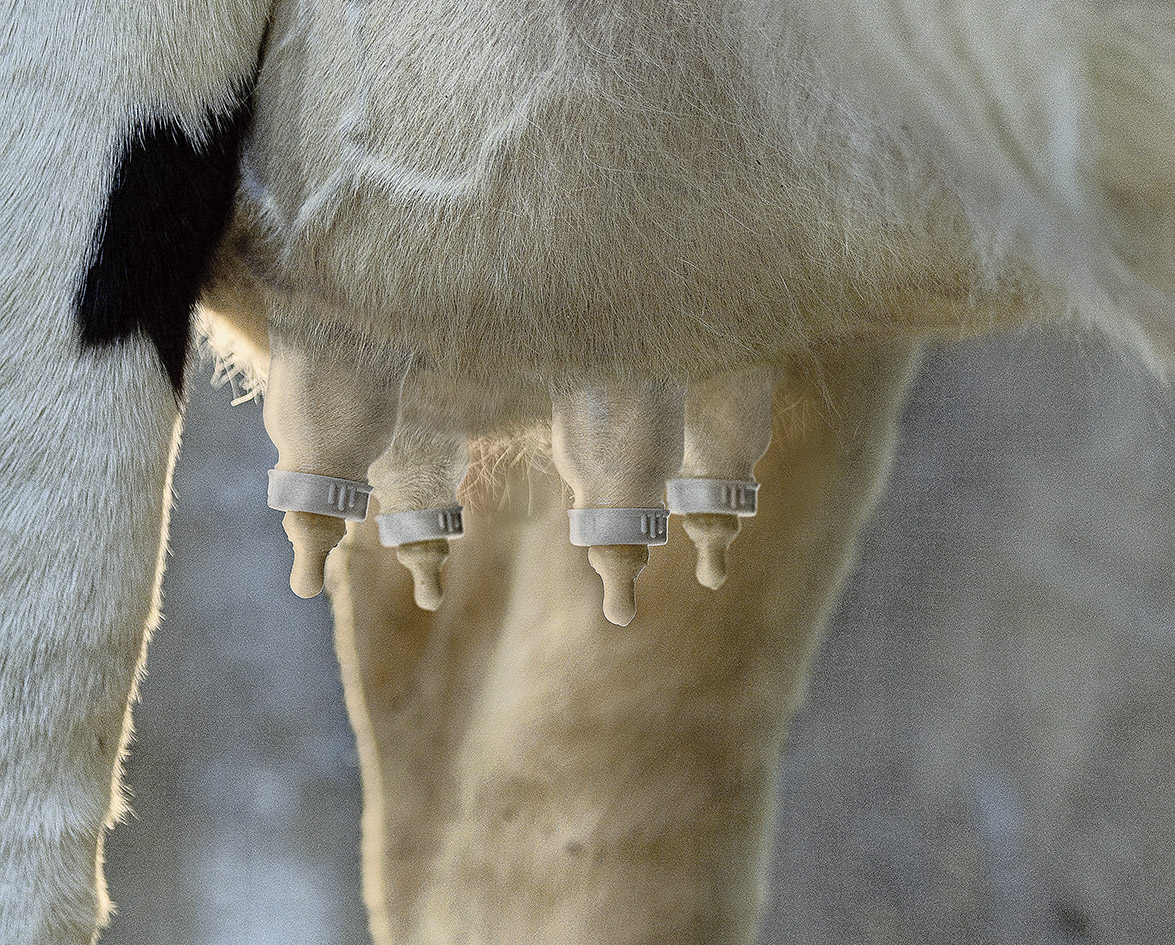
A new mom peruses the aisles of her local grocery store, picking up her family’s food for the week ahead. From the shelves she grabs the usual staples of bread, cereal and canned goods, along with new items such as baby food and diapers. She meanders over to the produce section, and finally to the dairy aisle to pick up eggs, cheese and human breast milk.
Playing God: Dairy Cows Human Breast Milk
In the future, finding human breast milk in your grocery store could be a reality. Through progress in biotechnology, the State Key Laboratory of Agrobiotechnology of the China Agricultural University has successfully developed genetically modified dairy cows: cows that are able to produce human breast milk. The transgenic herd of 300 Holstein cattle was developed through the process of inserting human genes into cloned cow embryos, which were then implanted into surrogate cows.

China began seeing low breastfeeding rates in the 1970s. Around the time they reached their lowest point to date, in the 1980s, breast milk substitutes such as infant formula were becoming widespread. Popular science was suggesting that breast milk substitutes were more nutritious than breast milk, based on the belief that rapid weight gain in infants indicates good health. China was operating a one-child policy—which is still in effect today—stimulating cultural pressure to ensure the health of the child.
The medical community now advocates breastfeeding. The World Health Organization and UNICEF introduced the Baby Friendly Hospital Initiative (BFHI) in the early 1990s. This initiative was a global effort, created to increase awareness of the benefits of breastfeeding to promote infant health. Before a maternity facility can be designated baby-friendly, it must accomplish a series of administrative and practical steps. A facility must create a breastfeeding policy and train all hospital staff. Baby-friendly hospitals do not accept free or low-cost breast milk substitutes, feeding bottles or teats. The initiative addresses factors that contribute to low breastfeeding rates, which include mother or child illness, mothers going back to work, breast problems, a dislike of or discomfort with breastfeeding, and, most commonly, perceived breast milk insufficiency. Despite the BFHI’s efforts, breastfeeding rates have not reached Chinese national targets.
The Debate Over Genetically Modified Products
Although genetic modification is not new, it continues to set off alarm bells around the world. So far, in North America, the only commercially passed genetically modified products for human consumption have been crops such as canola, soybeans, corn, and tomatoes. Genetically modified products are produced with the goal of growing larger crops, creating vitamin-enriched foods, and making vegetables resistant to herbicides, drought, and disease; but modifying living organisms comes with uncertainties.
You end up getting an animal with characteristics it wouldn’t necessarily have had before
Before a product is passed for public consumption a regulatory body must access it to determine its effect and stability within the ecosystem. Health Canada performs assessments in Canada, and the Food and Drug Administration (FDA) performs assessments in the United States. In China, however, there are more than ten government departments and ministries under the State Council that monitor food safety. These assessments must identify and recognize potential unforeseen effects on the ecosystem resulting from the manipulation of a species’ genetic structure. Effects can be difficult to control with crops because cross-contamination between genetically modified products and non-genetically modified products can occur through airborne pollination.
Genetically modified animals, whether for consumption or research, need to be examined for potential risks to the environment and human health as well as for ethical concerns of animal welfare. For ethical reasons, we need to understand if the process of developing genetically modified animals will harm the animals. Dr. Dan Weary, a Professor of Animal Welfare at The University of British Columbia (UBC), looks at the moral concerns: “In some cases, yes [animals are exposed to harm]. Procedures can be invasive, such as implanting embryos. Typically, there are many animals produced for one that is successful, so there is a lot of waste. Many more animals will be produced than are useful models.”
In the process of modifying one gene, another important gene could be affected, but this may not be realized until the process is complete and all experiments are finalized. In extreme cases the nature of the animal may be affected by mutagenesis (the process in which genetic information is changed, resulting in mutation) leading to unintentional harm. The Beltsville pig disaster of 1985 is a good example of mutagenesis leading to harm: genetically modified pigs were left blind and arthritic, with respiratory problems.

Graduate student Dr. Elisabeth Ormandy of the UBC Animal Welfare Program has focused her research on people’s attitudes toward genetically modified animals that have been created for scientific research. Dr. Ormandy explains, “Genetic modification is a random and targeted change in the genome of an animal, in the DNA of an animal, and whether that is an addition or deletion, substitution or manipulation of genes that are already present, you end up getting an animal with characteristics it wouldn’t necessarily have had before.”
She compares genetic modification to selective breeding. With cows as her example, she points out that we have been breeding cows for generations to produce high milk yields. This form of breeding can also have its side effects. Selectively bred cows have been infertile, developed mastitis, and become lame. Similarly, chickens have been bred to grow so large that their skeletons and muscles cannot support them and they are unable to stand.
Animals have been used in research for a variety of applications. Dr. Ormandy has conducted studies showing that people are more accepting of using animals for research than for genetic engineering. Survey results indicate that people generally oppose genetic modification of species for human consumption. “If it is going to go on your plate, people are not supportive of that by and large,” says Dr. Ormandy.
Enviropig—Environmentally Friendly, But Human Friendly?
Even so, scientists are seeing advancements in genetic sciences with the development of the Enviropig in Canada. Dr. Cecil Forsberg is a trained microbiologist and current professor emeritus at the University of Guelph. He has been a key player in the development of the Enviropig since 1998. The Enviropig was developed as an environmental solution to increased phosphorus pollution in areas of high swine production. Phosphorus feeds algae growth that leads to oxygen depletion in nearby waters, killing fish and emitting greenhouse gases. The source of this phosphorus pollution is found in pig feed. Phosphorus is a dietary mineral important in the formation of bones and cell walls as well as other organ functions. Pigs are not able to naturally digest phosphorus, and therefore excrete it. In heavy rainfalls the phosphorus-laden waste can spread to areas of fresh water. To address this problem, farmers can add the enzyme phytase to pig feed in order to aid pig digestion of phosphorus. This additive is an extra financial cost to the farmer.
The Enviropig has been genetically modified to produce phytase in pigs’ salivary glands, eliminating the need for the additive. When eaten by the pig, the pig feed mixes with the phytase, reducing the phosphorus content in the animal’s manure. This new, genetically modified process reduces phosphorus pollution—good news for environmentalists and farmers. But pigs are typically raised for human consumption, which begs the question: is the Enviropig edible?

Producers seek regulatory approval for their genetically modified products, but the question of public perception remains. Genetic modification fast tracks the testing period to gain quicker results than selective breeding, but do the potential risks outweigh its usefulness? In 2008, the International Food Information Council (IFIC) performed a survey of U.S. consumer trends with a focus on food biotechnology. The survey revealed that the majority of Americans have neutral feelings toward animal biotechnology. In questions where biotechnology offered special benefits such as improved animal health or nutritional quality, the results from the survey participants were positive. Many Americans are satisfied with the FDA’s policy on food labeling, and see no further information they would like added. The FDA uses special labeling on biotechnological products when a product’s nutritional content is affected or when an allergen is introduced. Similarly, the Asian Food Information Centre conducted a study in 2008 on consumer perceptions of food biotechnology in Asia. Chinese attitudes toward food safety in their country proved positive. Those surveyed emphasized expiry dates and vitamin nutritional information. There was little demand for biotechnology in food labeling. Awareness of food biotechnology is low, but those surveyed also felt that food biotechnology information would be beneficial in the next few years.
As more information regarding biotechnology and its uses becomes better known, it seems likely that science will continue to stride forward in the world of genetic modification. Barriers regarding the popularity of consuming genetically modified foods are individual and geographic. With the world’s population increasing, and with it the need for a larger food supply, it is indeed possible that genetically modified products—such as human breast milk—will one day be available to place in your shopping cart? Whether or not this is a step in the right direction is still up for debate.